Abstract
Introduction: The KIT D816V oncogene is a key driver in 90-95% of patients with systemic mastocytosis (SM), a group of mast cell (MC) neoplasms including indolent SM (ISM), smoldering SM (SSM) and AdvSM. Debilitating symptoms related to MC proliferation and degranulation characterize ISM and SSM and are also prominent in AdvSM, which is further complicated by SM-related organ damage and decreased survival. Currently, there are no approved agents that selectively target KIT D816V, and there are limited tools to assess symptom improvement in SM. Avapritinib, a highly potent and selective inhibitor of the KIT D816V mutant, showed substantial clinical activity in AdvSM (83% overall response rate (ORR) per modified IWG-MRT-ECNM criteria) in the Phase 1 EXPLORER study [Deininger, et al, EHA, 2018]. We present results using a novel PRO questionnaire, the AdvSM-SAF, developed in accordance with FDA guidance, to assess changes in symptoms in the Part 2 dose expansion phase of EXPLORER.
Methods: Patients (pts) received avapritinib at the recommended Phase 2 dose (300 mg once daily [QD]) in continuous 28-day (d) cycles. Pts completed the AdvSM-SAF and 2 additional PROs used in other cancers, the Patient Global Impression of Symptom Severity (PGIS) and the European Organization for Research and Treatment of Cancer Quality of Life (QLQ).
PROs and KIT D816V mutant allele fraction (MAF) in blood were assessed serially as follows: AdvSM-SAF daily, from 7 d before first avapritinib dose, ie, baseline (BL: Cycle [C]1 Day [D]1) and through C12, using an electronic diary; PGIS and QLQ at BL (C1D1) and on D1 of each cycle through C12; and KIT D816V mutant allele fraction (MAF) at BL and on D1 of C3, 7, 11, and then every 6 cycles.
The AdvSM-SAF assesses severity of 8 symptoms (pruritus, flushing, spots, nausea, vomiting, diarrhea, abdominal pain, fatigue) on a 0-10 scale, and 2 items assess frequency of diarrhea and vomiting. Results are analyzed as a Total Symptom Score (TSS), combining all 8 severity items (maximum symptom score=80), and as a GI domain (combining 4 symptoms: nausea, vomiting, diarrhea, abdominal pain; maximum score 40) and Skin domain (combining 3 symptoms: pruritus, flushing, spots; maximum score 30). Analyses were based on 7 d average scores. AdvSM-SAF data were summarized at BL (C1D-7 to C1D-1), and over the 7-d interval prior to C3D1 and C7D1, and correlated with QLQ, PGIS and KIT D816V MAF.
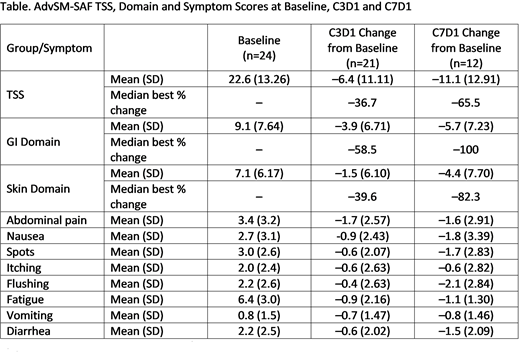
Results: As of 22 June 2018, 25 pts were treated in Part 2 of the study; 24 are ongoing, and enrollment and follow-up continues. The median duration of avapritinib treatment was 5.7 mo (range, 1.7+ to 10.3+ mo). AdvSM-SAF scores for 24 pts with data are shown the Table. Mean BL TSS, GI and Skin scores were 22.6, 9.1, and 7.1. The most severe BL symptom was fatigue (mean score of 6.4). Among 21 pts with scores at C3D1 and 12 pts with scores at C7D1, mean reductions from BL TSS, GI and Skin scores were 6.4, 3.9, and 1.5 points, and 11.1, 5.7, and 4.4 points, respectively, indicating further improvement with continued treatment. Symptom reductions were seen for all 8 items at C3D1. Further symptom improvement was observed for 6 of 8 items from C3D1 to C7D1.
Reductions in AdvSM-SAF TSS, GI and Skin scores significantly correlated with improvement in QLQ Emotional (EF) and Cognitive functioning (CF) scales (p values for Pearson Correlation Coefficients <0.0001, <0.0001, and 0.04 with EF and <0.0001, 0.0001, and 0.04 with CF, respectively). Among 18 pts with BL PGIS (mean 3.2, SD 1.15, range 1-5), improvement in PGIS was highly associated with improvement in GI score (p value 0.0004). Decreases in KIT D816V MAF correlated with reduction in TSS (p value 0.04).
Conclusions: Avapritinib treatment resulted in meaningful symptom improvement as measured by the AdvSM-SAF in overall symptoms (TSS), GI and skin domains, and all individual symptoms, and improvement continued with longer duration of treatment. Reduction in AdvSM-SAF scores correlated with improvements in other PRO instruments, PGIS and QLQ, supporting the AdvSM-SAF as a useful new tool to assess symptoms in pts with AdvSM. Reduction in AdvSM-SAF scores also correlated with decreases in KIT D816V MAF, indicating that reductions in disease burden may correlate with reduced disease symptoms and improved quality of life. These data warrant further development of avapritinib in AdvSM, as well as in ISM and SSM where symptom burden and poor quality of life are the predominant disease manifestations.
Gotlib:Deciphera: Consultancy, Honoraria, Research Funding; Blueprint Medicines: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Promedior: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Kartos: Consultancy; Incyte: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Research Funding. Radia:Novartis: Speakers Bureau; Blueprint: Consultancy. DeAngelo:Glycomimetics: Research Funding; Shire: Honoraria; Pfizer Inc: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Amgen: Consultancy; Novartis Pharmaceuticals Corporation: Consultancy, Honoraria; Takeda: Honoraria; ARIAD: Consultancy, Research Funding; BMS: Consultancy; Blueprint Medicines: Honoraria, Research Funding. Bose:CTI BioPharma: Research Funding; Celgene Corporation: Honoraria, Research Funding; Pfizer, Inc.: Research Funding; Blueprint Medicines Corporation: Research Funding; Incyte Corporation: Honoraria, Research Funding; Astellas Pharmaceuticals: Research Funding; Constellation Pharmaceuticals: Research Funding. Conlan:Blueprint Medicines: Employment. Oren:Blueprint Medicines: Employment. Shi:Blueprint Medicines: Employment. Deininger:Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Blueprint: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal